
Research groups
Colleges
Marissa Mueller
Postgraduate Student
Neurodevelopment, cortical layers 5 and 6b, neuroanatomy, electronics, bioengineering
Research Interests
Marissa's work investigates the role of the cortical subplate - a critical neurodevelopmental structure - as a substrate for the developmental origins of neurologic and psychiatric disease. She studies genetic knockdown models of the analogous murine cell population (cortical layer 6b) which result in conditionally increased (PTEN-silenced) and decreased (Snap25-silenced) subplate remnants through adulthood. In collaboration with the Charité-Universitätsmedizin Berlin (where Prof Molnár is an Einstein Visiting Fellow (2020-2024)), her project dissects how chronic perturbations influence brain physiology and pathology (e.g., epilepsy, narcolepsy, and autism). Her work investigates how the densities of subpopulations of cortical layer 5 and 6b cells differ across brain areas and genetic constructs. This broadly involves immunohistochemistry, microscopy, automated cell quantification, atlas regionalisation, and 3D brain reconstruction. These efforts seek to characterise unknown distributions and morphometrics of select cell populations, understand the effects of layer 5/6b manipulations, guide behavioural studies, and inform the development earlier disease interventions.
Biography
Marissa is from Petrolia, Canada. She earned her BSc in Engineering from the University of Iowa where she researched accelerometer algorithms and physical activity measurement. She completed the MSc in Neuroscience at Oxford with projects involving sleep physiology. This extended to a DPhil in Physiology, Anatomy, and Genetics. Outside academia, Marissa enjoys athletics, nature, travelling, board games, and music.
Neuroimaging
Collaborators
-
Zoltan Molnar
Professor of Developmental Neuroscience
-
Vladyslav Vyazovskiy
Professor of Sleep Physiology
-
Florina Szabo
DPhil Student
-
Auguste Vadisiute
Academic Visitor
-
Fernando Messore
Postdoctoral Research Scientist
-
Bashir Ahmed
Academic Visitor
-
Linus Milinski
Postdoctoral Research Scientist
-
Mona Barkat
DPhil candidate in Physiology, Anatomy and Genetics
-
Luana Campos Soares
Postdoctoral Research Scientist



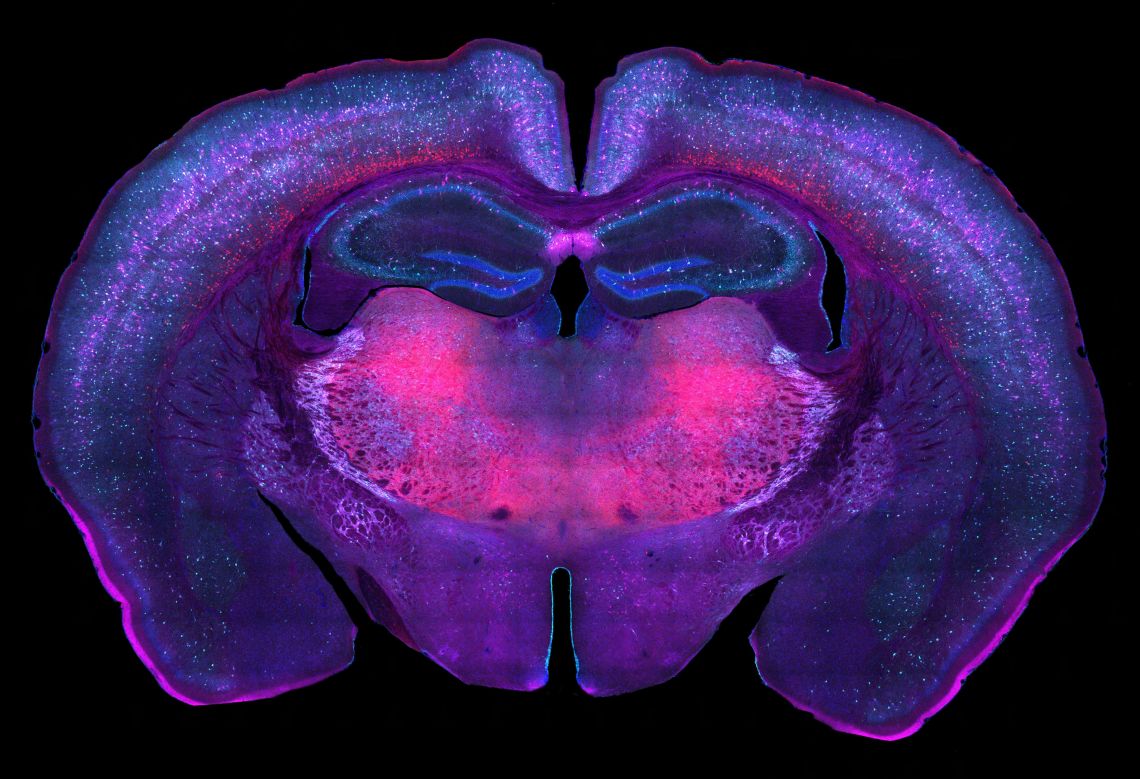 ©
Produced in collaboration with Florina Szabo, Oxford’s Cellular Imaging Core (WCHG), and the Eikholt Group (Charité University)
©
Produced in collaboration with Florina Szabo, Oxford’s Cellular Imaging Core (WCHG), and the Eikholt Group (Charité University)








