A collaborative study between the groups of Professor Juan De Carlos (Instituto Cajal, C.S.I.C., Madrid, Spain) and Professor Zoltán Molnár (Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford UK) has provided evidence for a new source of the earliest generated cerebral cortical cells, the subplate. In their recent publication in the journal Proceeding of the National Academy of Science USA (Pedraza et al., 2014) they describe that a considerable proportion of these early generated cells (known as the subplate) originate from an unusual and unexpected source, in the anterior and medial part of the telencephalon, outside the cerebral cortex itself. Previously, it had been generally accepted that they originate locally from the germinal zone of the cortical neuroepithelium.
These subplate cells in the embryonic cerebral cortex are particularly interesting for clinicians concerned with brain developmental abnormalities, since they provide a transient scaffold for the developing cerebral cortex and assist in the development of cortical connectivity, effectively providing the foundation of the adult structure. Indeed, the first synaptic contact in the cerebral cortex is established between subplate cells and the first thalamic fibers that reach the neuroepithelium. Disruption or damage to these early cells could be the source of flaws that lead to cerebral cortical developmental disorders, such as cerebral palsy, schizophrenia and autism. The discoveries that these scaffold cells arrive after a longer and more arduous journey might be significant to understand developmental disorders, because the process of their migration could be vulnerable and could be the cause of some cortical developmental abnormalities. If the scaffold is not assembled in the right time at the right place, then other aspects of the building work of making a brain can suffer which may only manifest in later life. This makes the discovery of this unusual origin and migration of the subplate neurons important for the developmental neurobiology and clinical neurology community.
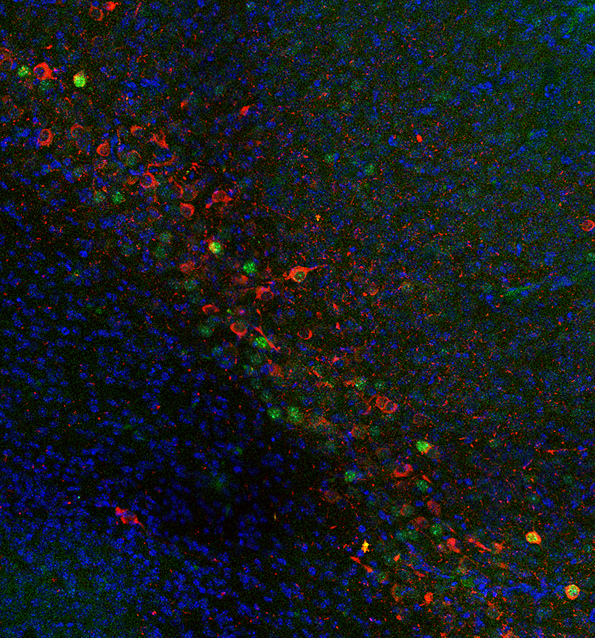
The team in Madrid, led by Professor De Carlos noticed that some subplate cells originate from an unusual anterior and medial location (rostral medial telencephalic wall) and migrate tangentially through long routes to the cerebral cortex. The team at Oxford studied a selective subgroup of subplate cells expressing Lpar1 (Edg2) which had similar distribution and characteristics to the labeled cells found by De Carlos and his team (Molecular diversity of early-born subplate neurons. Hoerder-Suabedissen A, Molnár Z. Cereb. Cortex. 2013 Jun;23(6):1473-83). When the two teams compared their results on a meeting they decided to use cell-tracker to label rostral medial telencephalic wall in the mouse strain with fluorescently labeled Lpar1-positive cells. These experiments showed a very good match and clearly demonstrated that indeed this subset of labeled subplate cells originated from this unexpected and unusual anterior-medial source in mice.
The layer of subplate cells is in the Cerebral Cortex of all mammals, but their size varies between species, being wider in humans and primates. This suggests that subplate cells are not a vestige of early neuronal structures, but a fundamental structure involved in early stage of cortical development. For this reason it will be important to study this source also in humans and hence, it will be imperative to study abnormalities of this particular subplate cell population in human cognitive disorders. It has been previously found that many of the schizophrenia and autism associated genes were expressed transiently in subplate during development (Expression profiling of mouse subplate reveals a dynamic gene network and disease association with autism and schizophrenia. Hoerder-Suabedissen et al., Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3555-60). Studying the way that nerves develop and connect this network can reveal where things can go wrong and trigger diseases such as the mentioned cerebral palsy, autism or schizophrenia.
This work was supported by the Spanish Ministerio de Ciencia e Innovación and by the Medical Research Council of the United Kingdom. Please visit the Proceedings of the National Academy of Sciences website for the full article (Pedraza et al., 2014).