Research groups
Websites
-
Kavli Institute for Nanoscience Discovery webpage
Kavli Profile
-
PubMed
Publications
-
ORCID profile
Publication list
-
OPDC
Oxford Parkinson's Disease Centre
Brent Ryan
Group Leader, Departmental Research Lecturer
- Research Scientist
Research Summary
My research focuses on identifying the earliest cellular phenotypes in Parkinson's disease and developing assays to measure these processes. These assays may be used to identify novel mechanisms or form the basis of high-throughput phenotypic and target-based screens to identify drugs to treat Parkinson’s.
In particular, my research interests are how the processes of mitochondrial dysfunction, oxidative stress and autophagy interact and influence cellular dysfunction in PD. To do this, we use a range of techniques and approaches including proteomics, the measurement of cellular bioenergetics and high-content imaging in three principle models:
- iPSC-derived dopaminergic cultures: With our collaborators in the OPDC, we collect skin cells (fibroblasts) from patients with Parkinson’s disease and reprogram these into induced pluripotent stem cells (iPSCs). We are then able to differentiate these cells into dopaminergic cultures, which we can use to understand the dysfunctions which cause these cells to be lost in Parkinson’s disease.
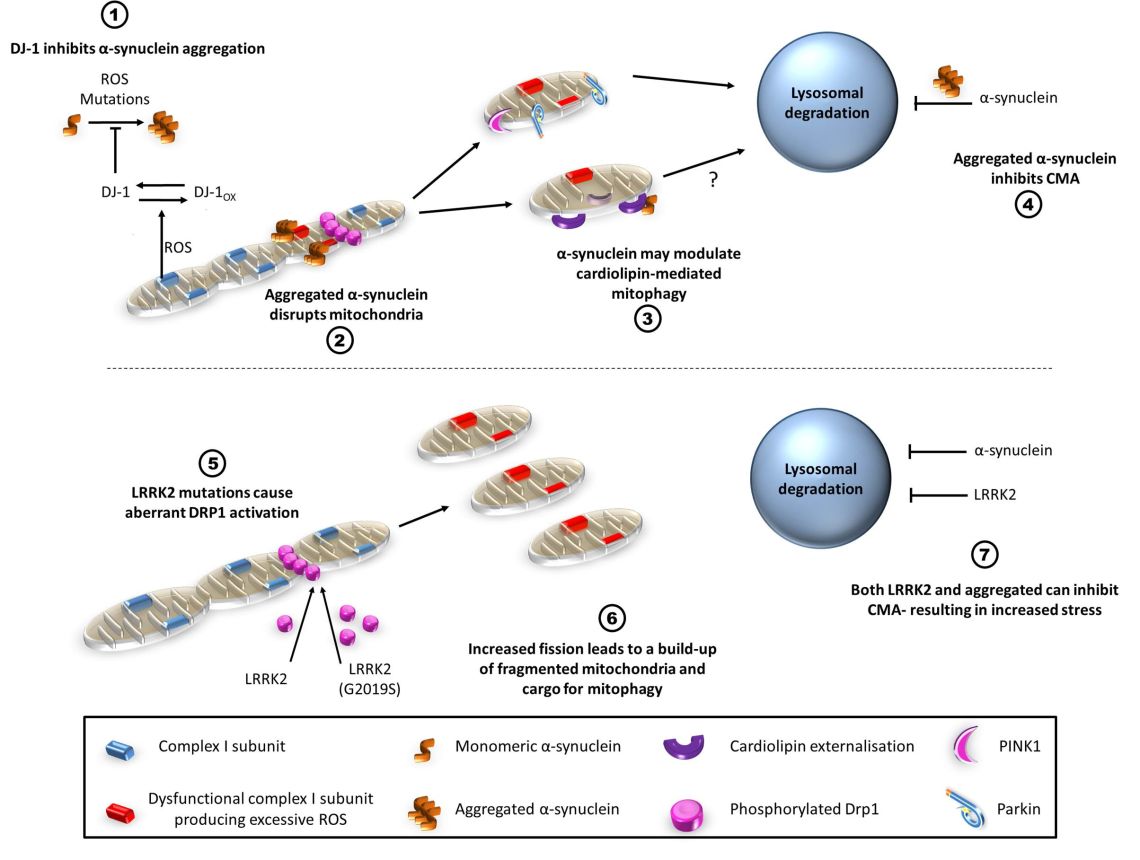
- α-synuclein BAC-transgenic mouse models: Our laboratory has previously generated a number of mouse models focusing on the expression of both wild-type and A30P mutant human α-synuclein in a physiologically regulated spatiotemporal manner. We are investigating how α-synuclein expression levels and mutations in α-synuclein affect mitochondrial function, oxidative stress and aggregation of proteins including α-synuclein.
- Toxin models of PD: Both PD-causing toxins MPP+ and rotenone cause mitochondrial dysfunction through complex-I inhibition and production of reactive oxygen and nitrogen species (ROS/RNS). We have investigated how these toxins alter the bioenergetics of these cells and the fundamental biochemical processes in cells. In addition, we investigate how levels of α-synuclein in the cell affect the dysfunctions induced by these neurotoxins.
Biography
I completed a PhD at University of Exeter (Peninsula Medical School) in which I studied the effects of oxidative post-translational modifications on the breakdown of immune tolerance in the autoimmune disease systemic lupus erythematosus. I joined the Wade-Martins lab in 2009 and initially worked on the effects of oxidative stress and mitochondrial dysfunction on cellular biochemistry in toxin models of PD and how these are impacted by α-synuclein levels. In 2015 I became an OPDC Career Development Fellow and am focussing on developing high-throughput screens for Parkinson's disease
Recent publications
-
TFEB and TFE3 have cell-type specific expression in the brain and divergent roles in neurons
Preprint
McGuinness W. et al, (2025)
-
Heritable maintenance of chromatin modifications confers transcriptional memory of interferon-γ signaling.
Journal article
Mikulski P. et al, (2025), Nat Struct Mol Biol
-
USP30 inhibition improves mitochondrial health through both PINK1-dependent and independent mechanisms
Preprint
Williamson MG. et al, (2025)
-
CRISPRi: a way to integrate iPSC-derived neuronal models.
Journal article
Franks SNJ. et al, (2024), Biochem Soc Trans, 52, 539 - 551
-
Correction: Multi-modal proteomic characterization of lysosomal function and proteostasis in progranulin-deficient neurons.
Journal article
Hasan S. et al, (2023), Mol Neurodegener, 18
-
Multi-modal proteomic characterization of lysosomal function and proteostasis in progranulin-deficient neurons.
Journal article
Hasan S. et al, (2023), Mol Neurodegener, 18
iPSC-derived dopaminergic culture
Roles of alpha-synuclein and LRRK2 in mitophagy